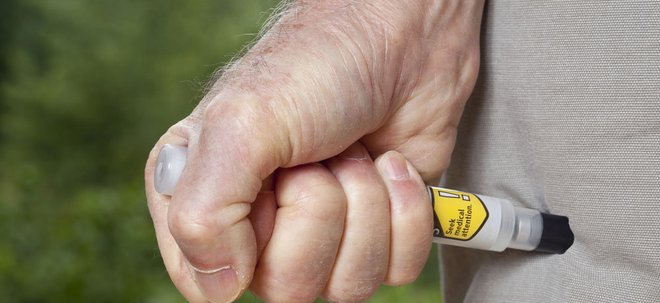
Mylan recalls 80,000 EpiPens
Drugmaker Mylan has recalled over 80,000 EpiPens across the world due to reports of the device failing to activate.
The recall comes after two instances of the device not working in emergency situations. The two people involved in the incidents were able to use an alternative EpiPen, AOL state.
EpiPen autoinjectors are devices that deliver doses epinephrine to people suffering from allergic reactions. If a device fails to activate it could be potentially fatal for the individual suffering from anaphylaxis symptoms.
According to Mylan the recalled devices could contain a defective part that is causing them not to activate.
The company recalled the devices out of concern for patient safety, Mylan state.
The potentially defective EpiPens expire in April. The company has released a list of the batch numbers that they state need to be replaced by users. Devices with these batch numbers can be replaced at a pharmacy for an unaffected device free of charge.