Operating expenses rise at Mainstay Medical as new trial begins
Medical device firm Mainstay Medical said operating expenses rose by $3.9 million for 2016 to $16.8 million as Mainstay kicked off its ReActiv-8 B clinical trial and began commercialising the product in Europe.
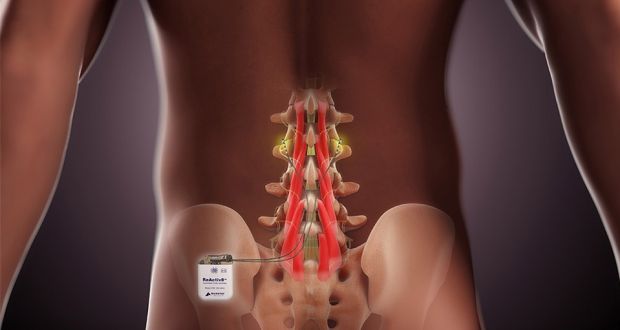
Publishing its annual report, the company, which makes an implantable device to treat chronic lower back pain, said it expected to see results from its latest clinical trial next year, paving the way for its entry to the US market.
Operating cash outflows were $16.7 million, with Mainstay reporting cash at hand on of $36.7 million by the end of December.
The ReActiv8 product is implanted in a surgical procedure and works by using electrical stimulus of nerves in muscles supporting the lower spine. Last year, it won European approval, leading to a €30 million share placement for the firm in June.
The company said it would initially concentrate on Germany as a market, implanting its first device in a patient there there in February.
It has now begun a clinical trial aimed at securing pre-market approval in the US, and implanted the first device in early October.
“Our ReActiv8-B Clinical Trial is on track and is a key step towards commercialisation in the US, our most significant target market,” said chief executive Peter Crosby. “Early in 2017, we began commercialisation in Europe, with the first sale and implantation of ReActiv8 in Germany, and look forward to gaining experience from our focused activities in this first market ahead of potential expansion to other territories.”
Written by Ciara O’Brien