Intriguing antitumor activity is found in a very promising class of natural compounds: cyclic depsipeptides, which have a challenging structure that makes their investigation difficult. Now, Chinese scientists have established
Drug Delivery

CN Bio Innovations Limited has announced today that it has entered into a Research Collaboration Agreement with the US Food and Drug Administration’s (FDA) Center for Drug Evaluation and Research
EGFR is a common genetic target in lung cancer, but not all EGFR mutations are created equal. Patients with a type of EGFR anomaly called an “EGFR exon 20 insertion”

Blood-thinning drugs not only reduce the risk of stroke in patients with atrial fibrillation (AF) but are also associated with a significant reduction in the risk of dementia, according to
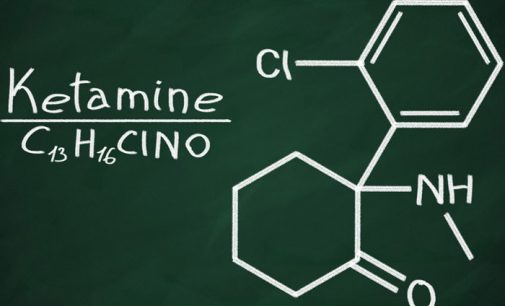
Ketamine, a medication commonly used for pain relief and increasingly used for depression, may help alleviate migraine pain in patients who have not been helped by other treatments. The study

The FDA has approved an expanded indication for Stelara for the treatment of adolescents with moderate plaque psoriasis. Janssen Biotech, Inc., has announced that the U.S. Food and Drug Administration
Titan Pharmaceuticals has announced that the first patient has been treated in a Phase I/II trial of its ropinirole subdermal implant for the treatment of the signs and symptoms of
A study by the U.S. Food and Drug Administration has backed up Aerie Pharmaceutical’s evidence that its experimental drug is effective in treating glaucoma. According to Reuters, the results were from a

IBS scientists at the Center for Self-Assembly and Complexity, within the Institute for Basic Science (IBS), invented a hydrogel to fight rheumatoid arthritis and other diseases. Published in Advanced Materials, this

University of Liverpool researchers, working with F2G Limited (Eccles, Manchester), have developed a new antifungal drug to help in the treatment of life threatening invasive fungal infections such as invasive aspergillosis.
The U.S. Food and Drug Administration is opening a new front in its efforts to reduce high drug prices by encouraging development of generic versions of hard-to-make medicines. Complex drugs

Camino Pharma, LLC, a new start-up company focusing on finding cures for cancer and brain disorders, announced that its Co-founder and Head of Drug Discovery, Nicholas Cosford, Ph.D., has been awarded
UCB has announced that the European Commission (EC) has approved expanding the use of its anti-epileptic drug (AED) Vimpat (lacosamide) as monotherapy and adjunctive therapy in the treatment of partial-onset
The UK National Institute for Health and Care Excellence (NICE) has granted approval for Roche’s breast cancer drug Kadcyla for routine use within the NHS region. NICE has recommended Kadcyla
Pfizer has secured approval the European Commission (EC) for TRUMENBA (Meningococcal Group B Vaccine) for preventing invasive meningococcal disease caused by Neisseria meningitidis serogroup B (MenB) in individuals 10 years

The European Medicines Agency (EMA) has approved Aptar Pharma’s integrated electronic nasal lockout device (e-Lockout) following a multi-year development with Takeda Pharmaceuticals International. Aptar Pharma agreed to supply Takeda with its
The European Commission (EC) has granted marketing authorisation for AstraZeneca’s Brilique (ticagrelor) orodispersible tablets (ODT) as a new method of treatment administration. This decision means that ticagrelor will become the
The European Medicines Agency (EMA) has approved Zebinix (eslicarbazepine acetate) for use as once-daily monotherapy to treat adults with newly-diagnosed partial-onset epilepsy. It is already indicated in Europe as an

The EC granted full marketing approval for Biogen’s Fampyra® (prolonged-release fampridine) as a treatment to improve walking in people with

Hansa Medical has accessed the EMA’s priority medicines scheme to accelerate the development of a therapy that broadens the access

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval to expand the use of
“New innovations in contraceptive technology are needed to expand the number of methods available to women and adolescents so they

Veltassa (patiromer), developed by Relypsa, has received a recommendation for marketing approval from the Committee for Medicinal Products for Human
Cinfa Biotech announced trial results for a biosimilar of Amgen’s Neulasta, a multi-billion blockbuster to treat chemotherapy-induced neutropenia. Cinfa Biotech

Clinigen Group’s Idis Managed Access division and Tesaro have partnered to launch a Managed Access Program (also known as
Pfizer has announced it will provide its breast cancer drug, palbociclib — which has been provisionally rejected by NICE for
EMA and the European Commission released a biosimilars information guide for health professionals during the EC’s biosimilars conference. In an
EU regulators have expanded the scope of Janssen’s Darzalex to include patients with multiple myeloma who have received at least
Impax Laboratories, Inc., a specialty pharmaceutical company, announced it has received final U.S. Food and Drug Administration (FDA) approval for a
BTG, a global specialist healthcare company, has received Class III CE Mark certification for DC Bead LUMI, the first commercially
Xeljanz (tofacitinib citrate) receives marketing authorization in the European Union for the treatment of moderate to severe active rheumatoid arthritis
Apeiron Biologics has announced that the EMA’s CHMP has recommended the approval of APN311 for immunotherapy of high risk neuroblastoma.
Newron, an Italian biotech focused on CNS diseases, is on a roll. The latest win? FDA approval of its Parkinson’s
Debiopharm International, a Swiss-based company, part of Debiopharm Group, announced that triptorelin 6-month formulation (Decapeptyl and Pamorelin 22.5 mg) received
Novartis has received FDA approval for Kisqali, a first-line treatment for breast cancer originally developed by the British biotech Astex
Innovus Pharmaceuticals, Inc., an emerging over-the-counter consumer goods and specialty pharmaceutical company engaged in the commercialization, licensing and development of
Italy adopts gender-neutral HPV vaccination program, citing study by health economics expert from Kingston University London’s Business School. The Italian
The National Institute for Health and Care Excellence (NICE) is half way through a review of drugs approved and still
Diagnostics company, Owlstone Medical, who is developing a breathalyser for disease has announced it has signed a master service agreement
Sanofi and its vaccines global business unit Sanofi Pasteur announced today an agreement with MedImmune, the global biologics research and development arm of
Amgen announced that the European Commission (EC) has adopted a decision to change the Repatha (evolocumab) marketing authorization, approving a new
GlaxoSmithKline plc and Innoviva, Inc. announced positive headline results from a non-inferiority lung function study, which demonstrated that patients with well-controlled asthma were
Mundipharma is gearing up to launch biosimilar Truxima in seven European markets for the treatment of certain cancers and inflammatory
The company has entered into a three-year agreement with Valeant Pharmaceuticals Ireland for the distribution in Europe, the Middle East
Researchers at Eindhoven University of Technology (TU/e) present a new method that should enable controlled drug delivery into the bloodstream
UK-based rare and speciality diseases group Mereo BioPharma says its brittle bone disease drug BPS-804 has been accepted to participate
Modus Therapeutics has raised €3.4M to support the completion of a Phase II trial for sickle cell disease, a painful condition
People living in Ayrshire and Arran who have some of the most common forms of avoidable sight loss will now